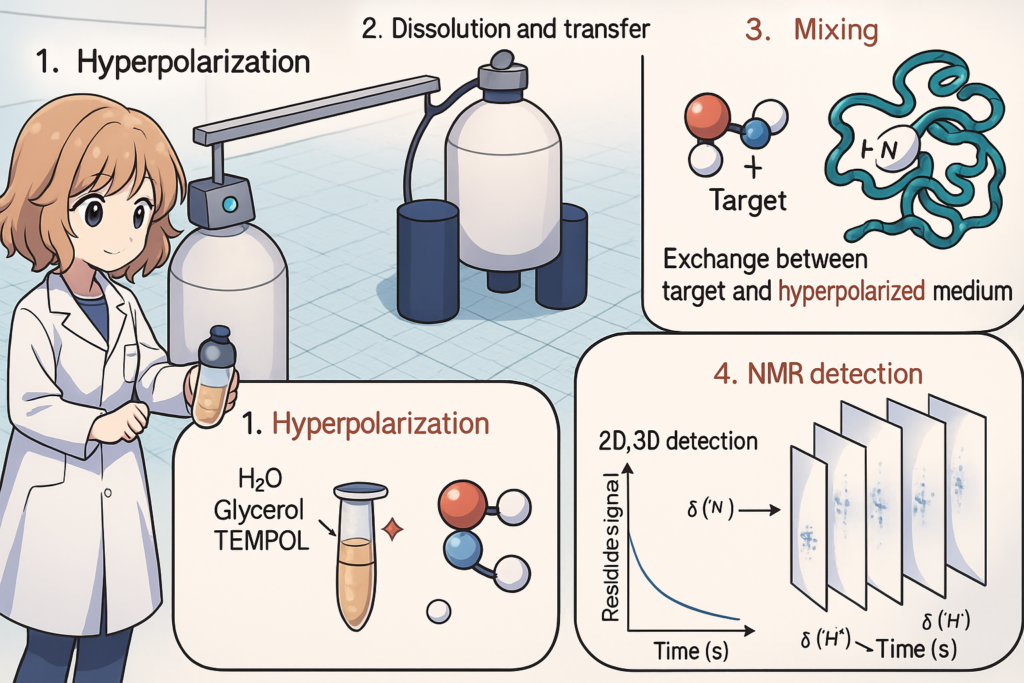
D-DNP for protein NMR
Our group leverages hyperpolarized water generated by dissolution dynamic nuclear polarization (dDNP) to overcome the intrinsic sensitivity limitations of conventional biomolecular NMR spectroscopy. By boosting the polarization of water protons prior to dissolving the biomolecular sample, we achieve signal enhancements exceeding four orders of magnitude, allowing for the recording of high-resolution spectra at physiological or even sub-physiological concentrations.
This approach enables the real-time detection of dynamic processes such as protein folding, intrinsically disordered protein dynamics, and solute–solvent interactions within seconds. Hyperpolarized water acts as a universal polarization medium, spontaneously exchanging protons with labile sites on biomolecules, thereby amplifying signals without the need for direct labeling or extensive sample preparation.
The integration of hyperpolarized water NMR with computational modeling and advanced spectroscopy (including EPR) allows us to capture transient conformational states, map folding landscapes, and elucidate biomolecular interactions with unparalleled time resolution and sensitivity.
Thus, hyperpolarized water serves as a versatile tool for studying complex biological systems under native-like conditions, opening new avenues for molecular mechanism discovery and rational biomolecular design.
Biomineralization and Biomimetic Materials
Our research integrates state-of-the-art hyperpolarization techniques with solution-state NMR spectroscopy to achieve molecular-level insights into biomineralization processes. By employing dissolution dynamic nuclear polarization (dDNP) and hyperpolarized water, we overcome the traditional sensitivity limitations of NMR, enabling real-time, residue-resolved monitoring of fast and transient events during mineral formation.
We investigate the early stages of crystallization by characterizing prenucleation species (PNS)—dynamic nanoscale ion assemblies that precede the solidification of materials such as calcium phosphate and calcium carbonate. Our studies reveal how branched polymeric PNS and other metastable intermediates drive non-classical nucleation pathways, challenging the classical nucleation-and-growth paradigm.
In parallel, we explore biomimetic self-assembly mechanisms, using silica-precipitating peptides to template the controlled growth of inorganic structures. Here, real-time hyperpolarized NMR enables us to capture fast solute-to-solid transitions, establishing direct links between peptide structure, assembly dynamics, and material morphology.
By combining hyperpolarized NMR, molecular dynamics simulations, SAXS, and cryo-TEM, we provide a unified atomistic view of biomineralization that connects solution-state dynamics with final material properties. These insights are crucial for the rational design of bioinspired materials with applications in medicine, energy storage, and environmental science.
Integrative Magnetic Resonance
Our research advances an integrative magnetic resonance (MR) framework that combines hyperpolarized NMR, electron paramagnetic resonance (EPR), and computational modeling to reveal dynamic molecular processes across multiple time and concentration scales.
Using dissolution dynamic nuclear polarization (dDNP) and hyperpolarized water, we dramatically enhance NMR sensitivity, enabling real-time tracking of transient states, reaction intermediates, and self-assembly events at native or near-physiological concentrations. This approach allows us to capture molecular phenomena such as biomineral precursor formation, protein conformational transitions, and early stages of material growth within seconds and at micromolar detection limits.
Computational techniques, including molecular dynamics (MD) simulations, docking, and free energy landscape exploration, are employed in parallel to provide atomistic interpretations of experimental NMR observations. This synergistic combination empowers us to connect structural dynamics with functional outcomes across scales.
To complement NMR-based investigations, we integrate EPR spectroscopy to characterize spin-active species—particularly paramagnetic centers involved in hyperpolarization processes or in biomineralization pathways. EPR provides critical insights into electron spin dynamics, radical behavior during hyperpolarization, and the local environments of paramagnetic agents, enhancing our understanding of both polarization transfer mechanisms and material precursor states.
By merging hyperpolarized NMR, EPR spectroscopy, and computational modeling, our integrative approach bridges experiment and theory, unlocking real-time, atomistic views of complex dynamic systems in chemistry, biology, and materials science.
See also:
